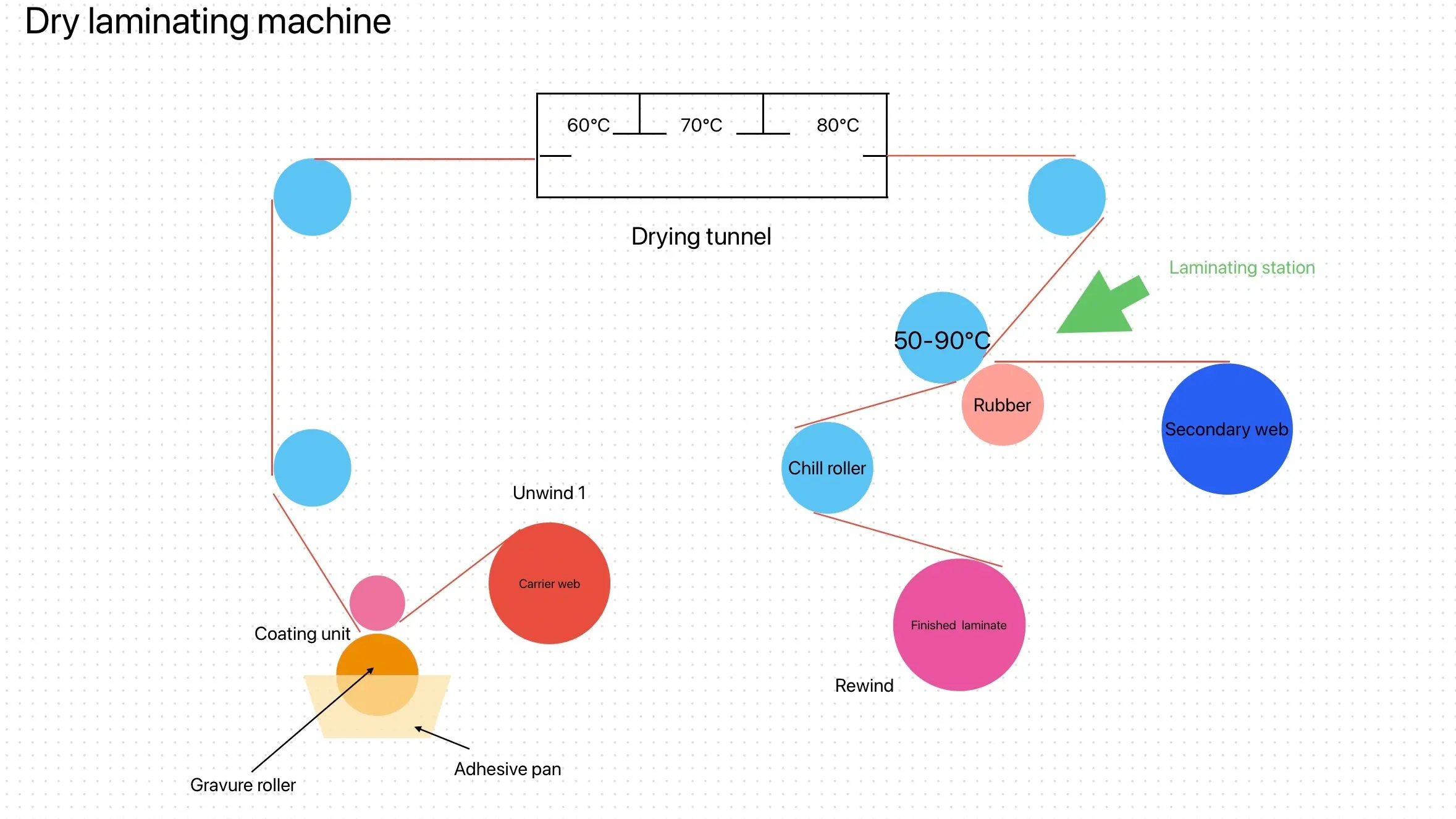
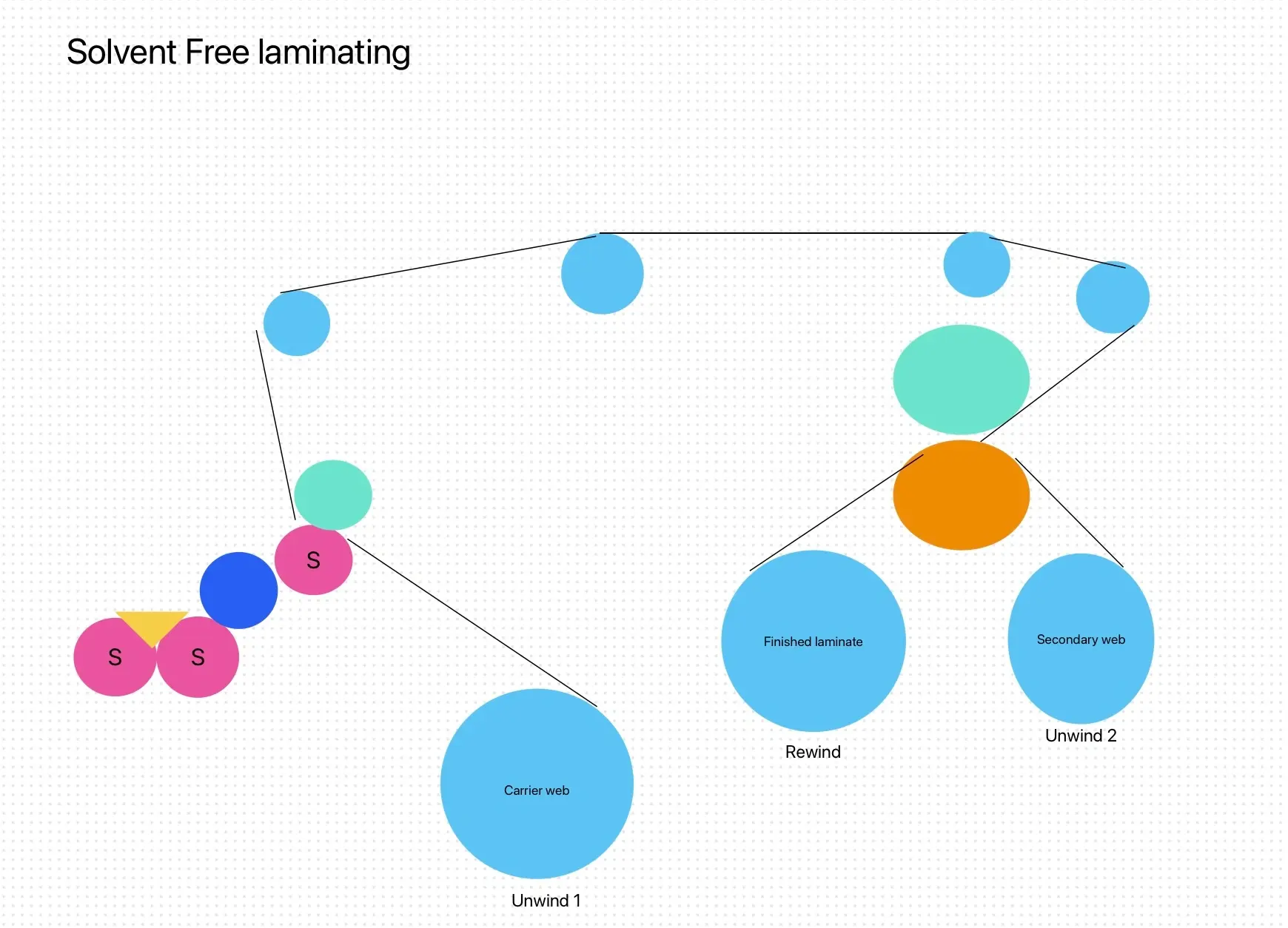
Solvent-based two-component polyurethane adhesives tailored for pharmaceutical packaging offer exceptional versatility and performance. They are designed for laminating a wide range of materials crucial in pharmaceutical applications, including PET, BOPP, CPP, PE, AL, NY, VMCPP, VMPET, and more. These liquid polyurethane adhesive are compatible with dry laminating machines operating at various speeds, ensuring flexibility and efficiency in production.
They are specifically formulated to withstand rigorous conditions such as 100℃/30min plastic boiling sterilization, meeting stringent pharmaceutical packaging requirements. With low solvent residue and excellent anti-attenuation properties, these adhesives maintain the integrity and safety of packaged pharmaceutical products. They also demonstrate superior performance in compounding PE structures, enhancing barrier properties and durability for pharmaceutical packaging that demands reliability and quality assurance.

For flexible packaging applications, adhesives need to meet several criteria:
Regulatory Compliance: Adhesives used in food packaging must comply with relevant regulations regarding food contact materials to ensure safety.
Compatibility: They should be compatible with the materials used in flexible packaging, such as various films, foils, and laminates.
Barrier Properties: Adhesives should contribute to maintaining the barrier properties of the packaging to protect the contents from moisture, oxygen, and other contaminants.
Application Method: Depending on the production process, adhesives may need to be applied via techniques like extrusion coating, lamination, or as a cold seal.